Title: Upcycling Single-Use Polyethylene into High-Quality Liquid Products
Authors: Gokhan Celik, Robert M. Kennedy, Ryan A. Hackler, Magali Ferrandon, Akalanka Tennakoon, Smita Patnaik, Anne M. LaPointe, Salai C. Ammal, Andreas Heyden, Frédéric A. Perras, Marek Pruski, Susannah L. Scott, Kenneth R. Poeppelmeier, Aaron D. Sadow, Massimiliano Delferro
Journal: ACS Central Science
Year: 2019
Article: https://pubs.acs.org/doi/10.1021/acscentsci.9b00722
Cover images courtesy of pixabay’s MatthewGollop and Skitterphoto
Plastics surround us in our lives; they are used in packaging of food and personal care products, electronics, and even our clothing and furniture! Over 75% of these products are single use or have a short lifespan which means that we are generating huge amounts of plastic waste. This plastic does not break down quickly, and ends up littering our cities, rivers and oceans. To clean up this mess, we need a new solution since recycling programs are not effectively capturing all the plastic waste. The researchers here have found one: converting plastics into a valuable product that can be sold for a profit. Since plastics are made of oil, a catalyst can break down and convert the plastic back into liquid products.
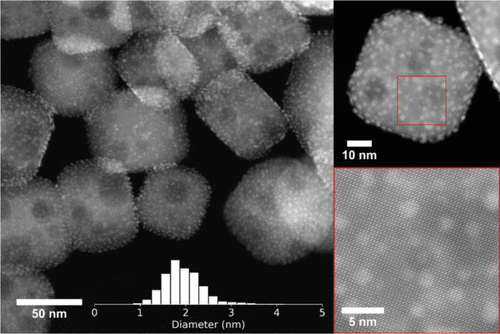
Scientists have developed a catalytic process to convert plastics, made of polyethylene, into high quality liquid products, such as lubricants and waxes. This has been done using platinum nanoparticles on a base of SrTiO3 perovskite nano-cubes (Figure 1). A perovskite is a type of material that has the same crystal structure of calcium titanium oxide. Scientists have developed ways to make other types of perovskite structured materials that are useful for a variety of applications.
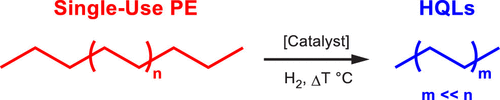
The chemical reaction that is occurring here is hydrogenolysis, which involves adding hydrogens onto long chains of carbons to break apart the C-C bonds (Figure 2). A major challenge faced by these researchers is to have the hydrogenolysis occur on select C-C bonds, so that the product liquid is quite pure, and doesn’t require extensive refining to be useful.
Researchers developed this new catalyst and tested its viability in reacting with single use plastics. While performing these reactions they studied the changes in the weight of products versus time to understand what reactions were occurring and how long they took. They also measured the conformation, or the shapes, that the plastics took when they were adsorbed to the solid catalyst. Lastly, they determined the strength of the adsorption of the plastic on the facets of the nanoparticles to study how the reaction differed on the edge sites. This was done using nuclear magnetic resonance and computational modeling to determine what conditions preferentially lead to certain products of interest. From this analysis they were able to determine where the active sites of the catalyst are, the metal nanoparticle. They also found that the size of the nanoparticle influences the molecular weight of the products that are observed from catalyst.
This more effective recovery of plastic material would be the equivalent of recovering $175 billion of oil that can be put into new industries instead of creating greater pollution. This is an exciting technology that could be more successful than current plastic recycling programs, which rely on down-cycling plastics: usually involving shredding the plastic and using the threads to make a new product. Better recycling, and less dependence on plastics and oil, are necessary to overcome our current global plastic pollution.