Title: Tailoring Kidney Transport of Organic Dyes with Low-Molecular-Weight PEGylation.
Journal: Bioconjugate Chemistry
Authors: Du, Bujie; Jiang, Xingya; Huang, Yingyu; Li, Siqing; Lin, Jason C.; Yu, Mengxiao; and Zheng, Jie
Year: 2019
https://pubs.acs.org/doi/10.1021/acs.bioconjchem.9b00707
Featured image and figures reproduced with permission. Further permissions related to this material must be directed to the ACS.
In recent years, many scientists have investigated nanoparticles—particles whose size range from 1/300th to 1/30,000th the width of a human hair [1]—as novel drugs or drug delivery systems. For example, silver nanoparticles are being investigated as antibiotics [2]. In addition, many nanoparticles are being investigated for cancer treatment because nanoparticles accumulate more in cancerous tissue than healthy tissue, due to tumors having a leakier blood vessel system (so more nanoparticles get to the cells) and a less effective drainage system away from the cells (so nanoparticles will stick around for longer) [2]. Nanoparticles are desirable due to their physical properties in the human body, but many of these properties have not been systematically investigated. One important question is: how does nanoparticle size effect the body’s ability to excrete the nanoparticle in the urine? Medicine needs to stay in the body long enough to work, but all medication has side effects, so doctors don’t want drugs that will stay in the patient’s body for too long. The scientists in this work, Du et al., decided to study the renal clearance—the speed at which a drug is removed from the blood via the kidneys—of polyethylene glycol (PEG) nanoparticles.
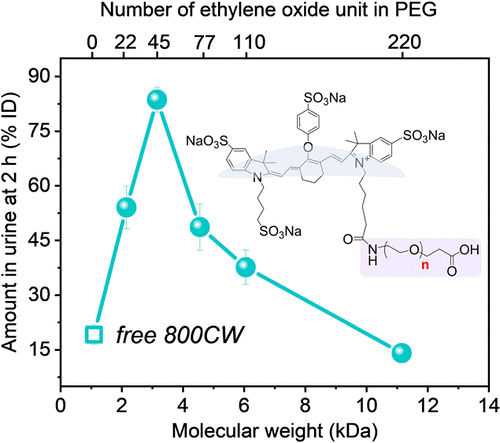
PEG nanoparticles are often used for drug delivery because they are compatible with the human body and have good mixture of water solubility (which is important for blood circulation) and fat solubility (which is important for getting drugs through the lipid cell membrane). Also, the size of a PEG nanoparticle can be controlled by the number of ethylene oxide units in each PEG molecule (Fig. 1). For example, PEG22 has 22 ethylene oxide units, whereas PEG220 has 220. It is generally believed that smaller particles have faster renal clearance because smaller particles would more easily be able to exit the blood vessels into the kidney tubules. However, Du et al. found in previous experiments that gold nanoparticles used in cancer treatment actually have an optimal size for renal clearance: a 1 nm-wide cluster is optimal, but renal clearance worsens exponentially as the number of gold atoms is reduced. However, gold nanoparticles are hard, metal objects, and PEG nanoparticles are soft, and thus might have different physical properties in kidney filtration.
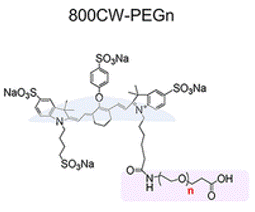
To track the renal clearance of differently sized PEG particles, the scientists attached PEG molecules to an organic dye called IDye800CW (800CW) (Fig. 1) which emits light at near-infrared (NIR) wavelengths. 800CW was chosen because—like PEG—it is also cleared from the body via renal filtration and excretion in the urine. Additionally, since 800CW emits NIR light, the scientists could use NIR detection to visualize the location of the dye inside a live mouse. In their experiment, the scientists found that, compared to free 800CW, 800CW-PEGn had faster renal clearance in mice for most lengths of PEG tested. Like the gold particles, there seemed to be an optimal PEG length for renal clearance—PEG45—and both longer and shorter PEG molecules led to slower clearance (Fig. 2A). Images of a mouse (Fig. 2B) 1 to 30 minutes after intravenous injection of either 800CW or 800CW-PEG45 showed that the free dye spread at high concentrations throughout the mouse’s body, whereas 800CW-PEG45 was localized largely in the kidneys and only spread at low concentrations through the rest of the body. Additionally, by 30 minutes most of the 800CW-PEG45 had moved from the kidneys into the bladder (Fig. 2C). This shows that while the free 800CW dye remained circulating in the blood, the 800CW-PEG45 was filtered into the kidneys and stored in the bladder for excretion.
Depending on the specific application of PEG nanoparticles in medicine, faster or slower renal clearance may be needed, either to prolong or shorten the effect of a drug on the human body. Systematic studies like this one by Du et al. could serve as useful references in the future for scientists looking to fine-tune the physical properties of their drug delivery system. The more we know about how the human body interacts with different kinds of nanoparticles, the more promising medicinal nanotechnology will be.
[1] https://www.reference.com/science/thick-human-hair-inches-ee40c0be6538b9ed
[2] Auría-Soro et al., Interactions of nanoparticles and biosystems: microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomaterials 2019, 9,1365.